lewis dot structure for nacl|NaCl (Sodium Chloride) Lewis Structure : Tagatay Learn how to draw the Lewis structure of NaCl, an ionic compound composed of sodium and chlorine ions. Follow the step-by-step guide and see the electron transfer, ionic bonding and . Pokémon Black 2 & White 2 are set within the Unova region 2 years after the events of Pokémon Black and Pokémon White, and as such, a lot has changed in the region. . First is the World Tournament which features many different tournaments featuring Gym Leaders and variousother powerful trainers.
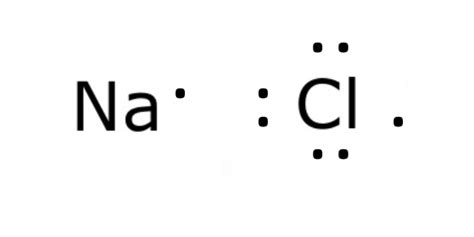
lewis dot structure for nacl,Learn how to draw the Lewis dot structure of NaCl, an ionic compound with a 1:1 ratio of sodium and chloride ions. Find out the crystal structure, polarity, and FAQs of NaCl. A step-by-step explanation of how to draw the NaCl Lewis Dot Structure (Sodium chloride).For NaCl we have an ionic compound and we need to take that into acc. A step-by-step explanation of how to draw the NaCl Lewis Dot Structure. For the NaCl Lewis structure, calculate the total number of valence electrons for the NaCl molecule. How to Draw the Lewis Structure of NaCl (sodium chloride, ionic) chemistNATE. 266K subscribers. Subscribed. 1.1K. 101K views 4 years ago Lewis Structures. Check me out:.Learn how to draw the Lewis structure of NaCl, an ionic compound composed of sodium and chlorine ions. Follow the step-by-step guide and see the electron transfer, ionic bonding and .This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha. Get the free "Lewis Structure Finder" widget for your website, blog, . Learn how to draw the Lewis dot structure of sodium chloride step by step, with examples and explanations. The web page also covers the octet rule, the ionic bond, and the key takeaways of Lewis dot structures.Lewis Structure of Sodium Chloride (NaCl) [ionic] 8.3.2024 Sodium is a metal in Group 1 of the periodic table, and therefore brings 1 valence electron with each atom: NaCl lewis’s structure is made up of one sodium (Na) atom and one chlorine (Cl) atom. The lewis dot structure of NaCl contains one positive charge on sodium (Na) metal . Steps to Draw the Lewis Dot Structure of NaCl. Step 1 : Determine the number of available valence electrons. In the periodic table, sodium is in the first group, and chlorine is in . The chlorine ion receives 1 electron, which are shown as dots in its outermost shell in the upper NaCl Lewis structure, and thereby attained stability by completing the octet. While the sodium atom only had one valence .Some old concepts such as Lewis dot structure and valency are still rather useful in our understanding of the chemical properties of atoms and molecules, and new concepts involving quantum mechanics of chemical bonding interpret modern observations very well. . Example \(\PageIndex{2}\): Ionic Bonding in NaCl. For example, in the reaction of .
Sodium, a metal in Group 1, loses one electron to become a +1 ionChlorine, a non-metal in Group 17, gains one electron to become a -1 ion.Together, they comb.

A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. . Chemists normally represent a bond using a line instead of two dots. The structures of H 2, F 2, and H 2 O would usually be drawn as follows:

Lewis structure of a water molecule. Lewis structures – also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs) – are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. [1] [2] [3] A Lewis structure can be drawn for any covalently .
Some old concepts such as Lewis dot structure and valency are still rather useful in our understanding of the chemical properties of atoms and molecules, and new concepts involving quantum mechanics of chemical bonding interpret modern observations very well. . Example \(\PageIndex{2}\): Ionic Bonding in NaCl. For example, in the reaction of . Drawing the Lewis Structure of NaCl. When we draw a Lewis dot structure, we aim to reach a state where all the atoms involved are stable, usually having full octets. We also strive to find a structure with the smallest possible formal charge. The general approach is to first identify all the elements involved along with their valence electrons .Lewis Dot Structure. Lewis dot structures reflect the electronic structures of the elements, including how the electrons are paired. Lewis structures are a useful way to summarize certain information about bonding and may be thought of as “electron bookkeeping”. In Lewis dot structures each dot represents an electron.lewis dot structure for nacl The Lewis dot structure for NaCl shows Na with one valence electron donating to Cl, which has 7 valence electrons. The resulting structure has Na surrounded by 8 electrons (full octet) and Cl .
Lewis Symbols. At the beginning of the 20 th century, an American physical chemist G. N. Lewis (1875–1946) devised a system of symbols—now called Lewis electron dot symbols (often shortened to Lewis dot symbols) that can be used for predicting the number of bonds formed by most elements in their compounds.Each Lewis dot symbol consists of the chemical symbol .
For period 2 elements, where all the valence electrons of an atom are in s and p orbitals, we find that the Lewis dot structure of molecules will often follow the Octet Rule:. Octet Rule - Atoms tend to gain, lose, or share electrons until they are surrounded by eight electrons (4 electron pairs).. Using Lewis dot structures and the octet rule, we can predict and represent the . A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in . Calcium chloride lewis dot structure. Lewis dot structure of calcium (Ca) and chlorine (Cl) is a unique structure because it is an ionic compound formed by a metal (Ca) and non-metal chlorine (Cl) The Lewis dot structure of CaCl2 contains a 2+ positive charge on calcium metal and one negative charge on chlorine nonmetal.. As calcium is placed in the 2nd .The structure of dot representation for the formation of NaCl is written above. Was this answer helpful? 43. Similar Questions. Q1. Draw electron dot representation for the formation of sodium chloride. View Solution. Q2. Draw electron dot representation for the formation of .lewis dot structure for nacl NaCl (Sodium Chloride) Lewis StructureA Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.Question: Draw the Lewis Structure for NaCl. Draw the Lewis Structure for NaCl. Here’s the best way to solve it. 100 % .
A step-by-step explanation of how to draw the AlCl3 Lewis Dot Structure (Aluminum chloride).For the AlCl3 structure use the periodic table to find the total .NaCl (Sodium Chloride) Lewis Structure Na (sodium), a metal, loses one electron to become a +1 ionCl (chlorine), a non-metal, gains on electron to become a -1 ion.Together, they form NaCl, one uni.
lewis dot structure for nacl|NaCl (Sodium Chloride) Lewis Structure
PH0 · Understanding the Lewis Structure of NaCl
PH1 · Sodium chloride (NaCl) lewis dot structure, polar or nonpolar,
PH2 · NaCl Lewis Structure: How to draw the Lewis Dot Structure for NaCl
PH3 · NaCl (Sodium Chloride) Lewis Structure
PH4 · Lewis structure of NaCl
PH5 · Lewis Structure of Sodium Chloride (NaCl) [ionic] – ChemistNate
PH6 · Lewis Structure Finder
PH7 · Lewis Dot Structure for Sodium Chloride
PH8 · How to Draw the Lewis Structure of NaCl (sodium chloride, ionic)
PH9 · How to Draw the Lewis Dot Structure for NaCl: Sodium chloride
PH10 · 10.3: Lewis Structures of Ionic Compounds